A Non-profit Organization to Help Students Study, Explore, and Engage
Naming Inorganic Compounds: Try a new formula
Click here to see the Legend. See also: typical oxidation numbers
| Is the formula charged? | ||
  |
||
| No | Yes | |
 |
 |
|
| Continue below | Is the atom on the right side an Oxygen? | |
|
|
||
 |
||
 |
Leave this page, this formula belongs to a different template. |
  |
|||||||||||
 |
 |
||||||||||
|
POSITIONING correctly within the formula: On the left is the  element with the
lower electronegativity.
This also means it has the lower group number (column) in the periodic table. element with the
lower electronegativity.
This also means it has the lower group number (column) in the periodic table. |
If
 or
or
 is
Oxygen
and the other is
Oxygen
and the other
then the position of the Oxygen is on the right side, i/e.  . . |
||||||||||
If
 and
and
 are in the same column in the periodic table, then the element that
is lower (larger atomic size) goes first (left) in the formula. are in the same column in the periodic table, then the element that
is lower (larger atomic size) goes first (left) in the formula. |
|||||||||||
| Continued: Each piece contributes a part of the name. | |||||||||||
 |
|||||||||||
 |
 |
||||||||||
| handle n | handle k | ||||||||||
 |
|
||||||||||
| n = 1 ? | The value of k always gets a Greek Numeral, even if it's 1. | ||||||||||
|
|
|
||||||||||
| Yes | No |
 |
|||||||||
 |
 |
 gets
its ionic name, gets
its ionic name, i.e. stem + -ide |
|||||||||
| Do not prefix with Greek numerals. Do
not say mono at the beginning!
|
is it Hydrogen |
 |
? |
 |
|||||||
 elemental name
(no prefix). elemental name
(no prefix). |
 |
 Yes Yes |
 No No |
Insert a space between the two
 components. components.
|
Greek Numeral +
ionic name as
 +
ide +
ide |
||||||
| n > 1
=> Prefix with Greek Numerals. |
|||||||||||
Greek Numerals +
 elemental name. elemental name. |
|||||||||||
Examples:
BrO2 = bromine dioxide <--- no mono at beginning
SiO2 = silicon dioxide
H2O = dihydrogen monoxide (this is the IUPAC name of water)
|
H2S = hydrogen
monosulfide
(IUPAC)§ |
<-- mono in the middle is ok §Additional names are available because H2S is also a binary acid. H2S= dihydrogen sulfide, dihydrogen monosulfide, hydrogen sulfide,and hydrosulfuric acid§ are used as well. |
|
N2O4 = dinitrogen tetroxide |
|
| N2O3 = dinitrogen trioxide | |
|
N2O = dinitrogen monoxide |
Commonly known as Nitrous Oxide. dinitrogen oxide is also an accepted name. |
| B2Br4 = diboron tetrabromide | |
| BrF5 = bromine pentafluoride | |
| AsH3 = arsenic trihydride |
Metaloid (As) Example Notice compounds with Hydrogen as the second  use
the name of the hydrogen ion: hydride. use
the name of the hydrogen ion: hydride. |
Compounds for which Common Names are prefered:
H2O2 = dihydrogen monosulfide,but hydrogen peroxide is often used instead.
O3 = ozone is used instead of the IUPAC name.
P4 = red phosphorous is used instead of the IUPAC name.
Paying attention to the charge...
SPECIAL CASES:
SO3 = is a neutral, covalent gas (at room temperature).
| is SO3 an
|
SO3 = sulfur trioxide, is based on the current naming scheme.This is so because SO3 is not charged. | |
| How about SO32- ? | SO32- = is a solid crystal lattice ion
in aqueous solution. It is negative and pairs up with cations in ionic
compounds. SO32- is a You must notice the charge. This is a The naming scheme is different than the current one. It is based on oxyanion naming with a baseline of n= 4. SO32- = sulfite. |
|
All compounds involving non-metals are essentially covalent with the exception of NH4+, in which case the bond is ionic.
Covalent compounds have no net charges, and can have no ions in them. See caveat.
The
Identical atoms bond to each other (diatomics)
Notice that the
Greek Numerals
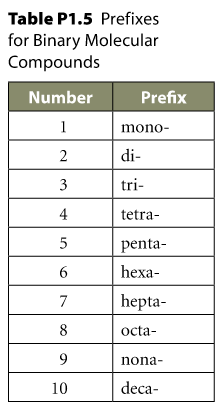
Greek Numerals Use
Never duplicate your "o"-s when naming:
for ex.
mono + oxide is not monooxide, but rather monoxide.
When a name forms "a" + "o", the "a" can be dropped but it's not mandatory. For example:
tetra + oxide
penta + oxide
For ex., pentaoxide and pentoxide are both correct. The dropping of the "a" is the more common form.
Academix: Study, Explore, Engage...
Copyright © 2025 Academix. All Rights Reserved.